Dear readers:
My name is Nadia Gadi and this is my first blog for the InnovEOX project. I am the ESR4 of the European H2020 MSCA-ETN InnovEOX project, for more details about my background and project please click on this link : https://innoveox.eu/esr4-nadia-gadi.
As part of the InnovEOX team, my project aims to the design of a novel full scale reactor for micropollutants removal from water using an electro advanced oxidation process at industrial scale. My challenge is to investigate and solve the main limitations for the scale up, and get by the end of this project to a reactor that will be efficient for micropollutants removal from water.
Why is there a pollution in treated water?

Sweden Water Reaserch
Water is one of the most important resource for humans, animals and plants life. Apart from drinking it, humans need water for different other uses such as the agriculture, industries, domestic use… The demand of this precious resource keeps increasing with the growth of the population over the world. According to the United Nations World Water Development Report of 2018, the water stress will affect around 52% of world’s population by 2050. Another issue that is faced with the water resource is its quality. Indeed, a large variety of chemicals and pollutants are identified in surface and ground waters1. Although high amounts of pollutants are removed by the conventional wastewater treatment plants (WWTP)2, the micropollutants are still present in the effluents. This shows a flaw in the efficiency of the WWTP and the urge to find new technologies to eliminate the refractory compounds from the treated water.
Some questions can come to mind: Were the micropollutants always been present in the effluents? Haven’t they been detected before because the analytical technologies were not as developed as they are today? The answer can be yes, because the methods used during the conventional treatment which are physical treatment followed by the biological treatment are still the same. Without the current stringent water regulation and standards, we would probably have never had the acknowledge of the existence of these compounds in treated water. In the other hand, the emergence of micropollutants in water can also be related to the increasing input of these component in water. Indeed, it was estimated that currently more that 70 000 of different chemical products are produced by the industries2,3. Hence, the pharmaceuticals and personal care products, pesticides and many other chemical products are in continuous and increasing input to the environment than what it was few years ago.
Micropollutants in drinking water from source to the tap- Method developement and application of a multiresidue screen method
How can these micropollutants be removed from water?
The priority now is to develop novel and sustainable processes for a total elimination the micropollutants from water. Indeed, various techniques are already being investigated and tested at lab and pilot scale, some of these methods such as the advanced oxidation processes (AOPs) showed promising efficiencies4. Due to their ability to generate non selective and very powerful oxidants such as the hydroxyl radicals (HO*) in the water, the AOPs have attracted a lot of attention. Thanks to the HO* generation (Eq 1-4), the AOPs are able to eliminate a large range of organic compounds even at low concentrations. 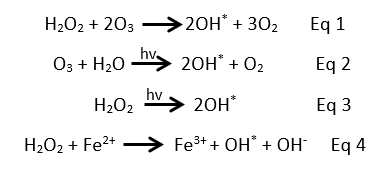 The elimination efficiency of micropollutants in water using the AOPs is directly related to the concentration of the generated HO*, which in its turn depends on the added amount of H2O2. However, the required H2O2 reagent can be too high depending on the concentration of the pollutants in water. Thus, inducing to larger treatment costs. Further, by considering the few nano seconds life time of the HO* reagent5, a continues generation of these radicals is required to ensure the oxidation process6.
The elimination efficiency of micropollutants in water using the AOPs is directly related to the concentration of the generated HO*, which in its turn depends on the added amount of H2O2. However, the required H2O2 reagent can be too high depending on the concentration of the pollutants in water. Thus, inducing to larger treatment costs. Further, by considering the few nano seconds life time of the HO* reagent5, a continues generation of these radicals is required to ensure the oxidation process6.
Therefore, alternative methods such as the Electro-Advanced Oxidation processes (eAOPs) are investigated for a continues in-situ generation of the HO radicals, H2O2 together with many other oxidants for a better cost effectiveness. Thus, several studies are focused on the development of efficient electrode materials for the electogeneration of H2O2 and HO*. So far, the graphite felt, carbon felt, graphite and activated carbon felt exhibited high efficiencies for cathodic H2O2 generation, as well as for the production of HO* at the anode using a Boron Doped Diamond material.
The H2O2 electrogeneration at the cathode surface and HO* at the anode is henceforth possible !
Nowdays, the challenge is to get to a chemical free processes for the elimination of the micropollutants from water by using less expensive anode materials.
The InnovEOX project aims to investigate and develop the new technologies for micropollutants removal from water steams using eAOPs and without producing any refractory or toxic byproducts. ( read more about it by following this link https://innoveox.eu/. My project focusses on the scaling-up of the eAOP for an industrial implementation. I will probably get to talk more about the applied eAOPs in my next blog !
(1) Zhou W, Rajic L, Chen L, et al. Activated carbon as effective cathode material in iron-free Electro-Fenton process: Integrated H2O2 electrogeneration, activation, and pollutants adsorption. Electrochimica Acta. 2019;296:317-326. doi:10.1016/j.electacta.2018.11.052
(2) Zhou MH, Lei LC. Electrochemical regeneration of activated carbon loaded with p-nitrophenol in a fluidized electrochemical reactor. Electrochimica Acta. 2006;51(21):4489-4496. doi:10.1016/j.electacta.2005.12.028
(3) Zearley TL, Summers RS. Removal of Trace Organic Micropollutants by Drinking Water Biological Filters. Environ Sci Technol. 2012;46(17):9412-9419. doi:10.1021/es301428e
(4) Vogelpohl A. Applications of AOPs in wastewater treatment. Water Sci Technol. 2007;55(12):207-211. doi:10.2166/wst.2007.408
(5) Rogowska J, Cieszynska-Semenowicz M, Ratajczyk W, Wolska L. Micropollutants in treated wastewater. Ambio. 2019;49(2):487–503. doi:10.1007/s13280-019-01219-5
(6) Garcia-Segura S, Keller J, Brillas E, Radjenovic J. Removal of organic contaminants from secondary effluent by anodic oxidation with a boron-doped diamond anode as tertiary treatment. Journal of Hazardous Materials. 2015;283:551-557. doi:10.1016/j.jhazmat.2014.10.003

Born and raised in Algeria, Nadia started her studies in fundamental chemistry at the University of Mouloud Mammerie Tizi Ouzou where she obtained her first Bachelor’s degree. After that, she moved to France and had an equivalence of her diploma in general chemistry after a year of study at the University of Versailles Saint Quentin en Yvelines.
Passionate with the environment field, especially by water treatment she completed a Master in Environment and Geomaterials at Gustave Eiffel University, followed by a research internship in the research laboratory of that same university (LGE) which focused on the electro-Fenton process for activated carbon fibers regeneration and pharmaceuticals removal from water. During this period, she was introduced to the eAOPs for water treatment at lab scale. At the end of the internship, she was hired for 8 months as a research engineer by LGE, for further exploration of the process used during her internship.
Motivated to take a step further and to work on eAOPs applied in wastewater treatment at industrial scale, Nadia is now an Early Stage Researcher at Nijhuis Industries (Netherlands) working on the ESR4 InnovEOX project “Reactor and process design for industrial implementation of eAOP” supervised by Dr. Nadine Boelee (Nijhuis Industries) and Prof. Raf Dewil (KU Leuven).
 InnovEOX Innovative Electrochemical OXidation Processes for the Removal and Analysis of micro-pollutants in water streams
InnovEOX Innovative Electrochemical OXidation Processes for the Removal and Analysis of micro-pollutants in water streams



