We are always looking for cheaper and sustainable materials irrespective of the industry or sector we work for, to develop new processes which are as cost-effective as possible. I am Rebecca Dhawle, a chemical engineer and ESR 06 based in University of Patras, Greece. And while digging through loads of research material, I found that biochar was being investigated as a cost-effective material in various applications. In my blog, I am going to tell you about biochar and its application in Advanced Oxidation Processes (AOPs).
Biochar is the carbonaceous material obtained by treating biomass such as leaves, wood and animal remains at high temperatures in the limited or no supply of air. The term “biochar” is derived from the Greek word bios and char meaning life and carbonaceous material respectively. Historically, the Pre-Columbian Amazonians produced biochar by smoldering, a process of covering burning biomass with soil. It is believed that they used it as a method to improve the soil fertility however, there is no proof to support this theory. A lot of research has been carried out on the applications of biochar ranging from soil health, reduction of greenhouse gases, waste management etc. The total number of research articles published per country from 1999 until 2018 is shown in the Fig 1. But what is of great importance is the use of these bio-derived charcoals in Advanced Oxidation Processes.
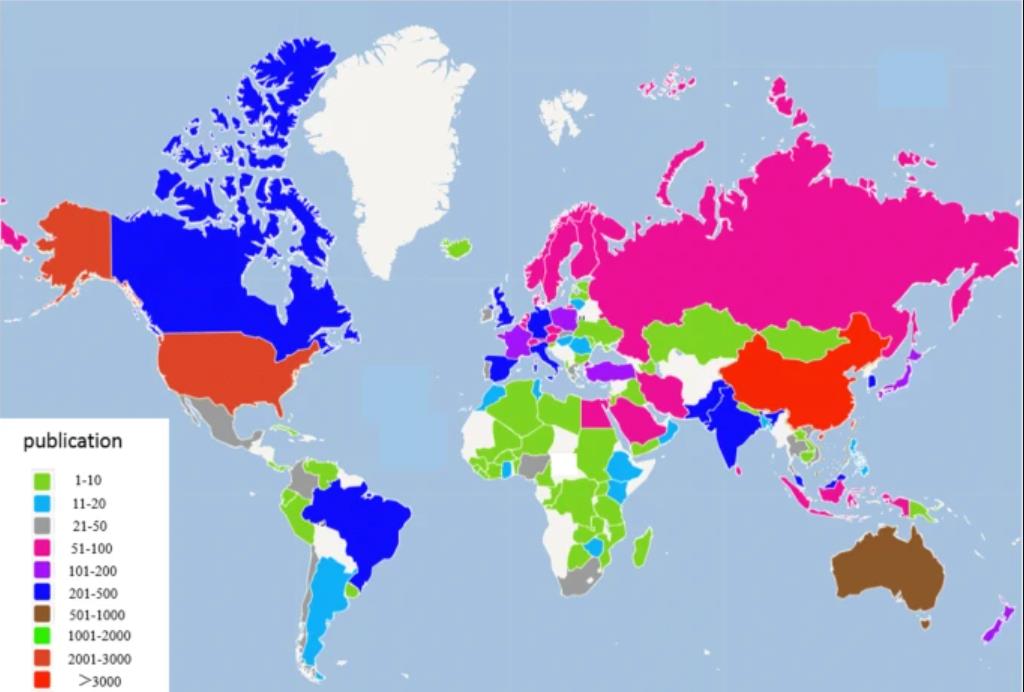
Figure 1: Number of research articles published on biochar in the period of 1999-2018 [1]
Advanced Oxidation Processes are the techniques employed for the removal or mineralization of persistent pollutants from water matrices. These pollutants usually found in low concentrations pose severe risks to human health and environment. With rising water quality standards and the demand for safe and clean water, the need for effective processes and materials for the degradation of these pollutants is imminent. Biochars have been investigated as a low cost and sustainable alternative to expensive catalysts.
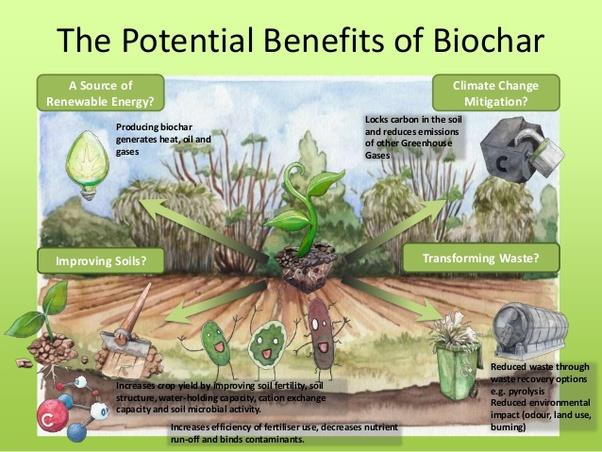
Figure 2: Benefits of biochar [2]
Biochar is a broad term, and a general characterization of biochar is not very easy. The quality of biochar depends mostly on the raw material that is used in the production. The cellulose, hemicellulose, lignin, moisture content, treatment conditions, origin of raw material and the preparation method determine the properties of biochar. However, despite these variety of characteristics, biochars have proven to be useful in a wide range of AOPs.
Due to their porosity, high surface area, active surface groups and conductive nature biochars are ideal to be used in numerous AOPs. Biochars have been used to generate OH radicals[3], activate persulfate[4] and peroxide[5] into reactive oxygen species (ROS). Biochars rich in metallic impurities have shown excellent results for Fenton like catalysts in the activation of hydrogen peroxide into hydroxyl radicals. This eliminates the pH limitation of the conventional Fenton process and the use of expensive metal catalysts. The use of biochars as a support for metal catalysts have also been studied. Not only do they help in providing a larger surface area for the
reaction to occur but also increase the efficiency of the enclosed catalysts by reducing the agglomeration of catalyst particles within the reaction mixture[6]. In some cases, a collective effect of the catalyst and biochar have also enhanced the efficiency of the degradation process. Apart from this, the use of biochars in the form of cathodes to catalyse electro-fenton processes has also gained a lot of popularity. These cathodes, in the presence of external ferrous ions, facilitate the activation of peroxide which is produced on-site[7]. Biochars employed in several AOPs have been successful in the removal of persistent pollutants such as sulfamethoxazole, ciprofloxacin, tetracycline and phthalates among others[8]. Simple modifications such as treatments with nitric acid[5], sodium hydroxide[9] and potassium hydroxide[10] tend to drastically improve the catalytic properties of biochar making them more beneficial in the treatment of contaminants.
Although the production of biochar may not be cheap depending on the process used to produce it and the energy consumption of the production process, they are proven to be relatively cheaper than the conventional catalysts used in AOPs. In comparison to activated carbon and other carbonaceous materials, the overall environmental impact of biochar is significantly lower. They have shown to retain their catalytic properties for more than three cycles demonstrating their stability and sustainability for future use[8]. Despite their numerous benefits, biochars also have minor disadvantages such as the dependence of their physicochemical properties on the production methods and the feedstock. The long term effects and the end fate of biochars used in these kind of processes is yet to be ascertained.
Let us hope that further research and studies would provide us with more answers on such questions and help overcome their limitations.
[1] D. Li, R. Zhao, X. Peng, Z. Ma, Y. Zhao, T. Gong, M. Sun, Y. Jiao, T. Yang, B. Xi, Biochar-related studies from 1999 to 2018: a bibliometrics-based review, Environ Sci Pollut Res. 27 (2020) 2898–2908. https://doi.org/10.1007/s11356-019-06870-9.
[2] Monique, Benefits, (n.d.). https://biochar.co.nz/benefits.
[3] X. Ruan, Y. Sun, W. Du, Y. Tang, Q. Liu, Z. Zhang, W. Doherty, R.L. Frost, G. Qian, D.C.W. Tsang, Formation, characteristics, and applications of environmentally persistent free radicals in biochars: A review, Bioresource Technology. 281 (2019) 457–468. https://doi.org/10.1016/j.biortech.2019.02.105.
[4] G. Fang, C. Liu, J. Gao, D.D. Dionysiou, D. Zhou, Manipulation of Persistent Free Radicals in Biochar To Activate Persulfate for Contaminant Degradation, Environ. Sci. Technol. 49 (2015) 5645–5653. https://doi.org/10.1021/es5061512.
[5] K. Luo, Q. Yang, Y. Pang, D. Wang, X. Li, M. Lei, Q. Huang, Unveiling the mechanism of biochar-activated hydrogen peroxide on the degradation of ciprofloxacin, Chemical Engineering Journal. 374 (2019) 520–530. https://doi.org/10.1016/j.cej.2019.05.204.
[6] A. Gopinath, K. Krishna, Photocatalytic Degradation of a Chlorinated Organic Chemical Using Activated Carbon Fiber Coupled with Semiconductor, Photochemistry and Photobiology. 95 (2019) 1311–1319. https://doi.org/10.1111/php.13130.
[7] P.V. Nidheesh, R. Gandhimathi, Trends in electro-Fenton process for water and wastewater treatment: An overview, Desalination. 299 (2012) 1–15. https://doi.org/10.1016/j.desal.2012.05.011.
[8] P.V. Nidheesh, A. Gopinath, N. Ranjith, A. Praveen Akre, V. Sreedharan, M. Suresh Kumar, Potential role of biochar in advanced oxidation processes: A sustainable approach, Chemical Engineering Journal. 405 (2021) 126582. https://doi.org/10.1016/j.cej.2020.126582.
[9] V.-T. Nguyen, T.-B. Nguyen, C.P. Huang, C.-W. Chen, X.-T. Bui, C.-D. Dong, Alkaline modified biochar derived from spent coffee ground for removal of tetracycline from aqueous solutions, Journal of Water Process Engineering. 40 (2021) 101908. https://doi.org/10.1016/j.jwpe.2020.101908.
[10] C. Wang, X. Li, W. Wu, G. Chen, J. Tao, Removal of cadmium in water by potassium hydroxide activated biochar produced from Enteromorpha prolifera, Journal of Water Process Engineering. 42 (2021) 102201. https://doi.org/10.1016/j.jwpe.2021.102201.

Rebecca is a Chemical Engineer from Bangalore, India. Since her school days she was fascinated by science and technology. She specialized in biology and chemistry during her high-school and then went ahead to get her Bachelor of Engineering degree in Chemical Engineering from the MVJ College of Engineering in Bangalore, India.
In 2017, she moved to Toulouse, France and pursued her MSc in Fluids Engineering for Industrial Process from the The Institut National des Sciences Appliquées de Toulouse (INSA Toulouse). The program focused on the physical analysis and modeling of coupled transfer mechanisms in multiphase flows. She had the opportunity to learn and use multiple CFD softwares during the Master’s program. She did her research internship at the Laboratory of Chemical Engineering, Toulouse. She worked on the “Modelling Of A Homogenous Photo-Catalytic Reactor For The Degradation Of Organic Pollutants Using AOPs”. This project kindled her interest in the field of advanced oxidation processes for water treatment.
Currently, Rebecca is a Marie Curie Early Stage Researcher (ESR6) in the InnovEOX project at the Chemical Engineering department of the University of Patras, Greece. She is working on “Hybrid Photoelectrochemical Processes” under the supervision of Prof. Dionisios Mantzavinos.
 InnovEOX Innovative Electrochemical OXidation Processes for the Removal and Analysis of micro-pollutants in water streams
InnovEOX Innovative Electrochemical OXidation Processes for the Removal and Analysis of micro-pollutants in water streams



